 |
| Here we are shown a PV graph and asked to find the W. |
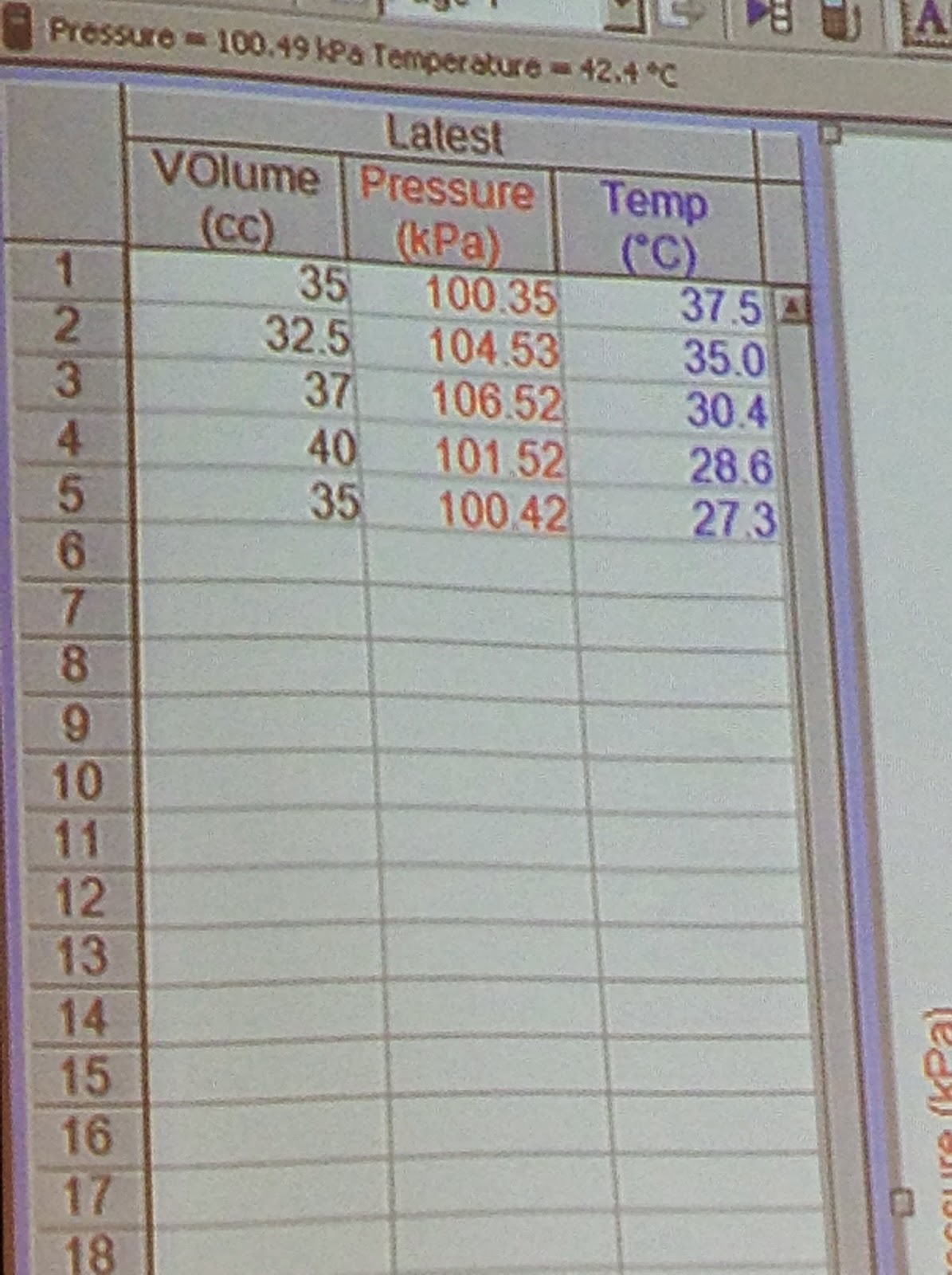 |
| Close up shot of Values |
 |
| Here we can use our definitions of internal energy work and heat, to solve for the constant Cp=5/2R. We can also do the same for Cv by letting work = 0 and Q=nCvdelT giving us Cv=3/2R Note: Cp-Cv=R |
 |
| Now we can show relationships between P T and V. by using our ideal gas law PV=nRT. Note: gamma = Cp/Cv |
 |
| Using our new Formula for Pressure initial we can now solve for W=Spdv. |







































.jpg)




